Optimized, Automated CE-SDS for Evaluating Oncolytic Coxsackievirus Capsids
Speaker: Mohamed Dawod, Ph.D., Senior Scientist, Vaccine Analytical R&D
Currently in Phase I/II clinical trials and classified as an investigational oncolytic agent,
Coxsackievirus A21 (CVA21) is being investigated against a range of solid tumors.
The mature (full) capsid consists of four viral proteins, VP1, VP2, VP3, and VP4, while immature (empty) capsids have only precursor VP0, VP1, and VP3 proteins.
Key challenges for successful scale-up of the manufacturing process include optimization of virus production, separation of empty and full capsids, and purification.
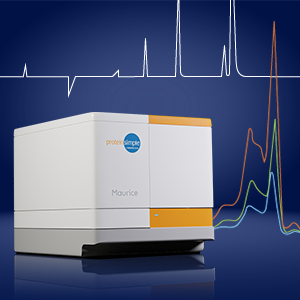
This portion of the webinar discusses the development of a CE-SDS method on a Sciex CE instrument for profiling viral proteins using a custom-made gel, followed by the development a CE-SDS method with optimized sample loading on a Maurice instrument from ProteinSimple.
The CE-SDS using Maurice provided acceptable signal intensity with baseline resolution for all 5 viral proteins.
The optimized method was qualified and applied for analysis of various process batches for profile and relative purity consistency.
In this webinar, you will learn about a faster, more efficient identity profiling and testing of downstream vaccine materials.
About the Speaker
 |
|
Mohamed Dawod, Ph.D.
Senior Scientist
Vaccine Analytical R&D
|
|
Mohamed Dawod is an expert in capillary and microchip electrophoresis.
With over 10 years of post-PhD work in academia and industry, his career has focused heavily on the enhancement of detection sensitivity and quantitative repeatability of capillary and microchip electrophoresis and microfluidic Western blotting.
Dr.
Dawod is a Senior Scientist in Vaccine Analytical R&D (VARD) and is the founder and lead of CE Center of Excellence within Merck.
He is also the co-lead of Scientific Excellence within VARD.
He has effective collaboration with peers and industrial partners in research grants and has generated more than 20 peer reviewed articles.
|
|